2020 Annual Report
#TEAMGENEVA
A Message from the President & CEO
ELISE HUSZAR, MBA
Geneva Fights COVID-19
2020 has demonstrated the critical importance of military medicine in shaping positive global health outcomes. Since the onset of the COVID-19 pandemic, Geneva researchers and employees have been relentless in pursuit of vaccines, treatments, therapeutics, and other novel solutions to address one of the most scientific challenges of our lifetime.
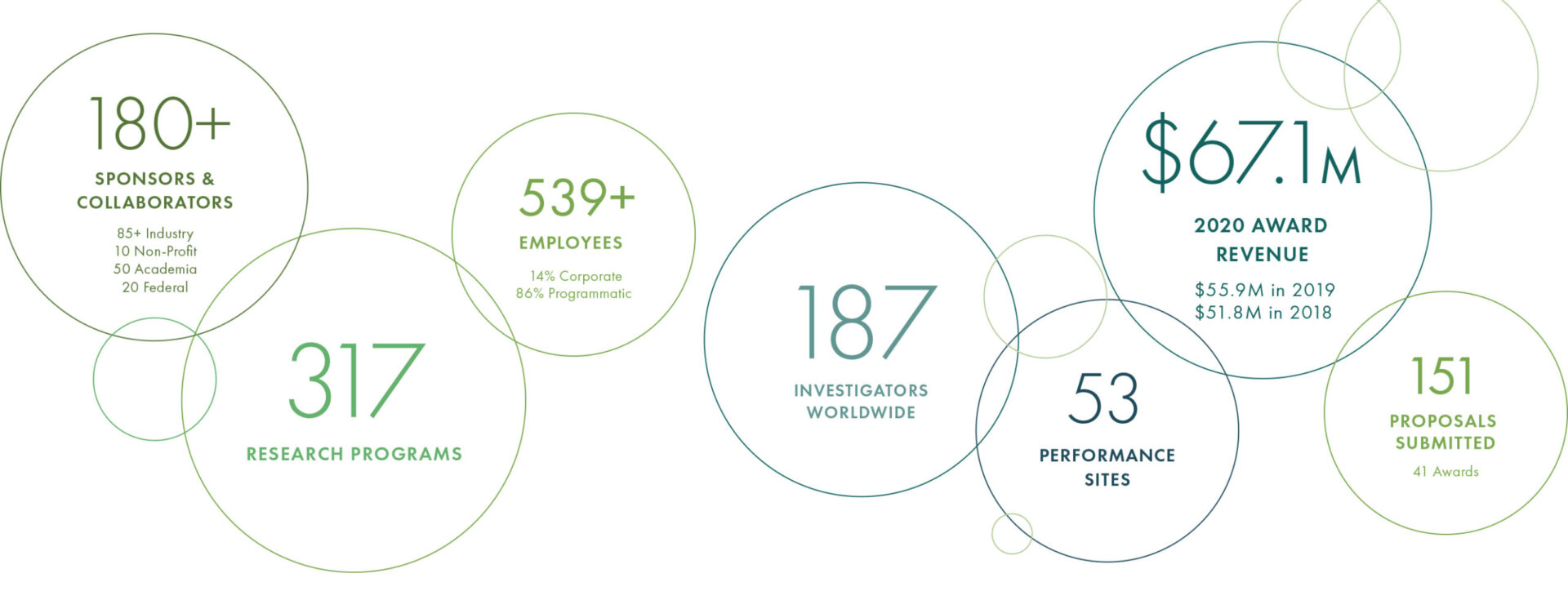
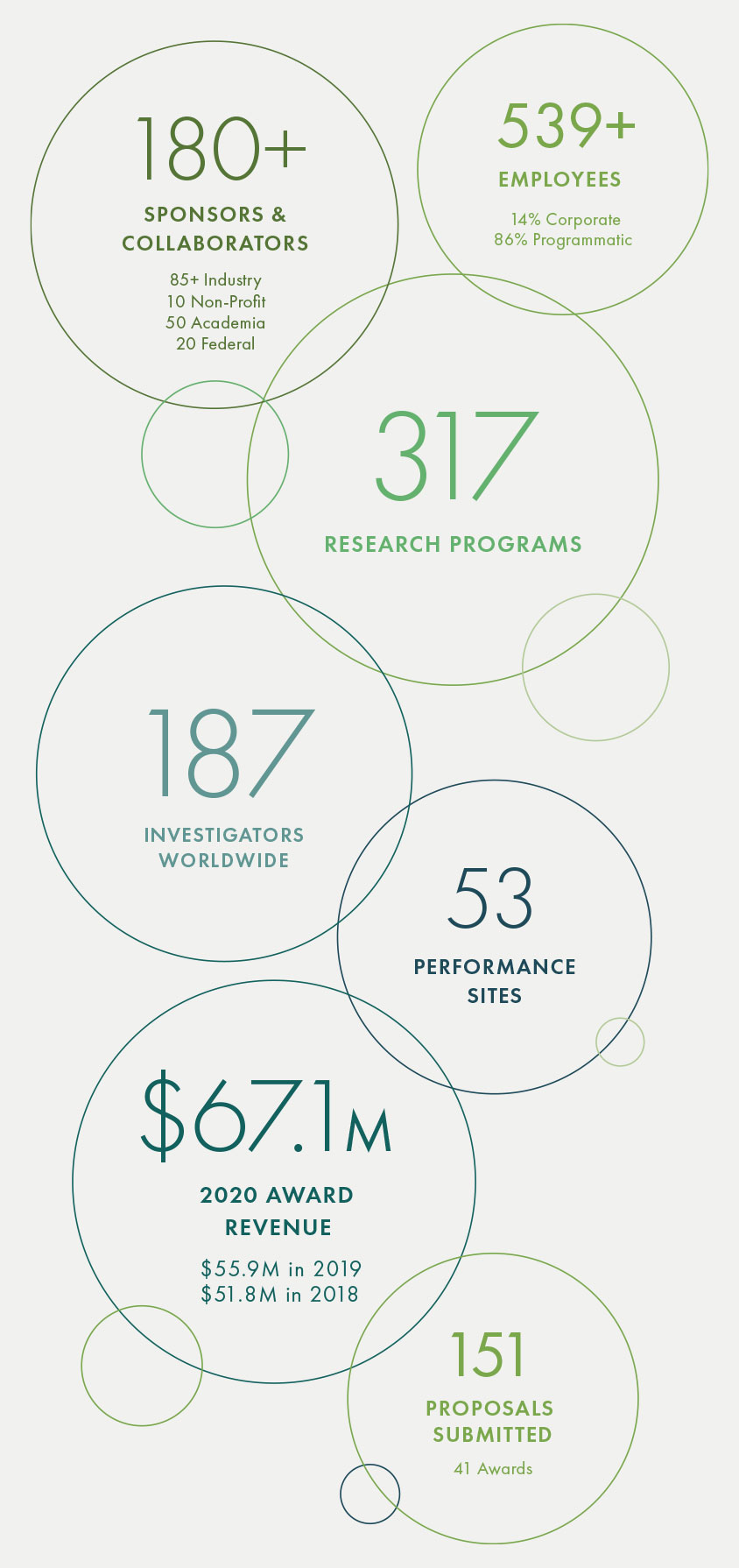
Read more2020 At A Glance
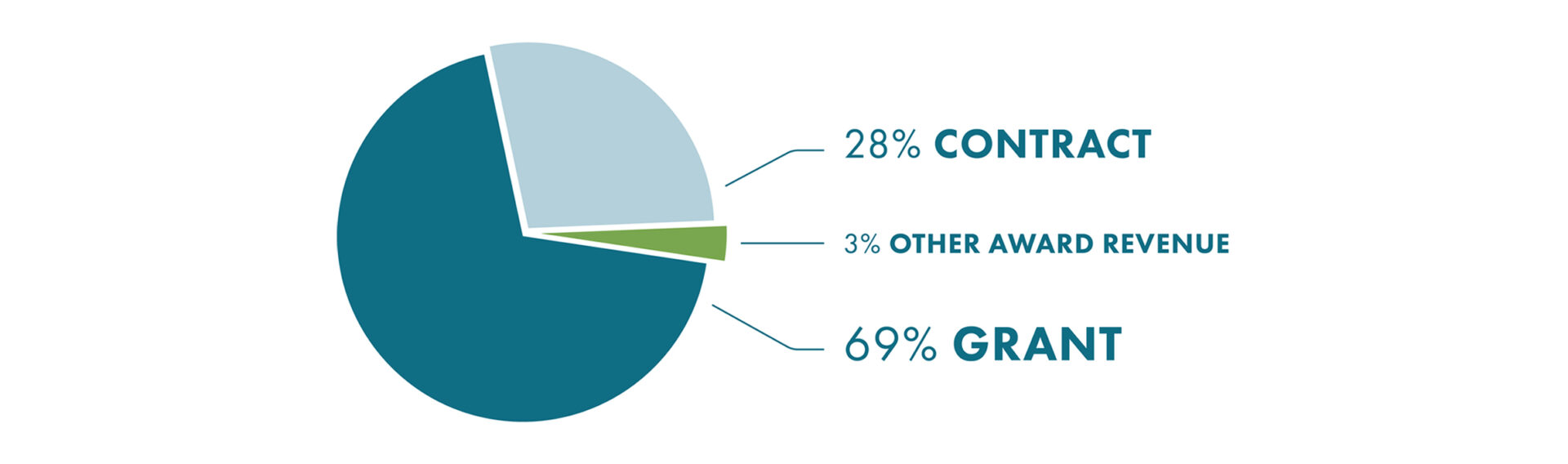
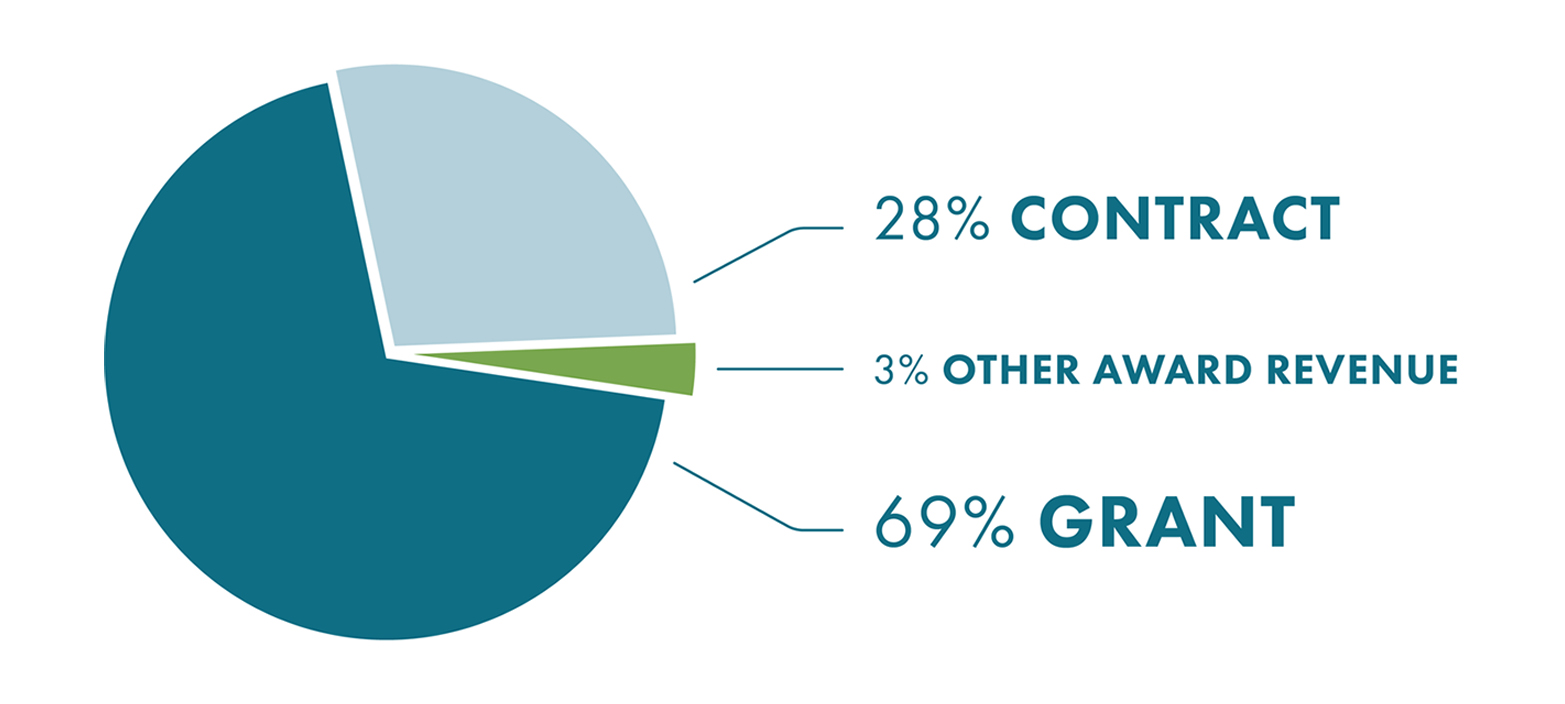
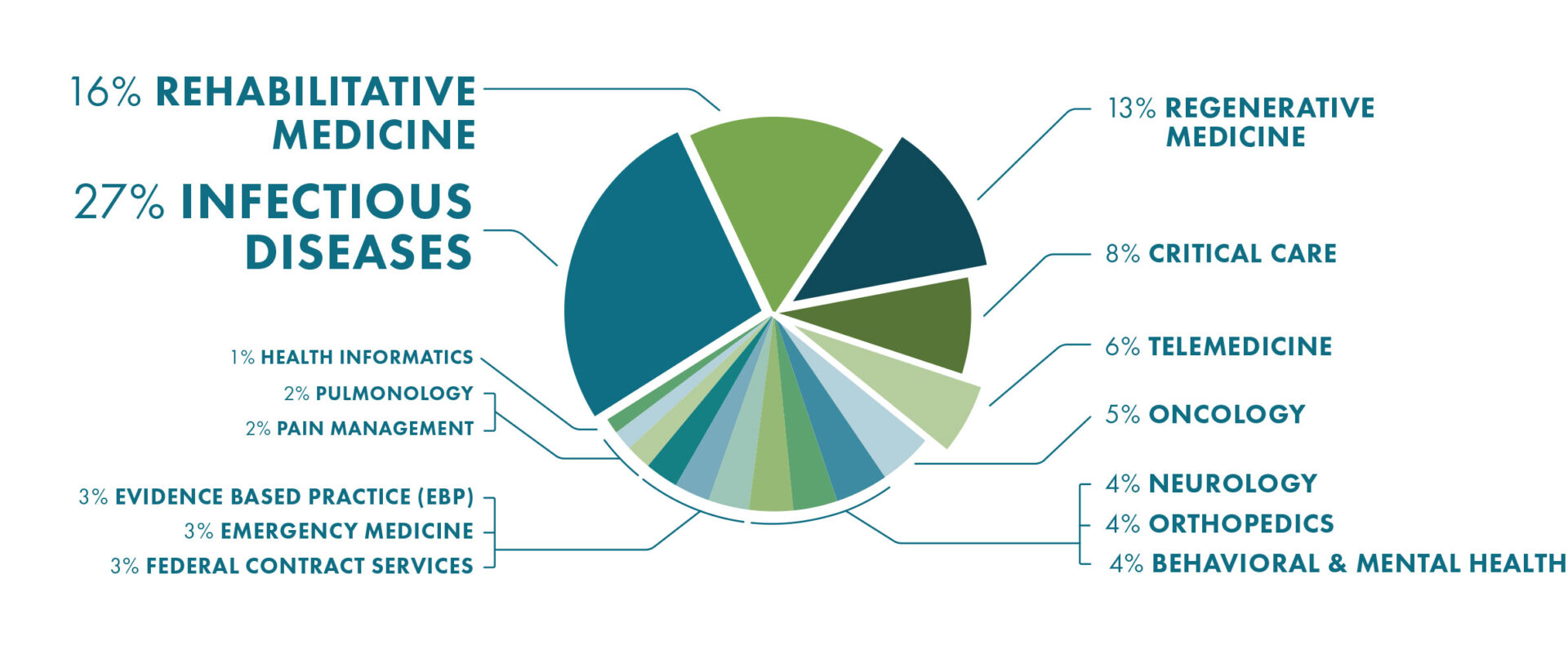
2020 Top 15 Research Programs By Funding

2020 Notable Achievements
February

Geneva launches the USU-4DBio3 Center for Biotechnology (USU-4DBio3 Center) On-Demand Blood Program to deliver on-demand blood and revolutionize military trauma care. This adaption for use near the point-of-need is a game-changer.
READ MOREMarch
Geneva researchers John M. Dye, PhD and Andrew Herbert, PhD of the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), along with their team, develop the first-ever experimental antibody treatment that protects from Sudan virus.
READ MOREApril

Geneva hosts the first-ever Post-Operative Rehabilitation Meeting to standardize 13 musculoskeletal injury protocols for the Army, Navy, and Air Force. This significant meeting was made possible by the support of the Broussard Family Foundation.
READ MORE
In partnership with USU, TechShot, Inc, and nScrypt, Geneva successfully completes the first 3D printing test experiment of a human knee meniscus onboard the International Space Station U.S. National Laboratory.
READ MOREMay
The Food and Drug Administration (FDA) issues an Emergency Use Authorization for Vekliry (remdesivir) for the treatment of hospitalized patients with severe coronavirus disease. Geneva researcher and employee Travis Warren, PhD and his colleagues are among the first scientists to run early tests on remdesivir as a treatment for Ebola virus.
READ MORE
Geneva receives a subaward for an expanded phase of the CARE Consortium. As part of the NCAA-DoD Grand Alliance, it is the largest study in history of concussion and repetitive head injuries with the goal to identify ways to improve diagnosis, treatment, and prevention.
READ MORE
The FDA authorizes emergency use of the COVID-19 Airway Management Isolation Chamber (CAMIC) within the military for use by healthcare providers to protect against exposure to airborne particulates during transport of COVID-19 patients. The CAMIC was developed by a team of Army doctors including Geneva researcher MAJ (P) Douglas Ruhl, MD, MSPH at Madigan Army Medical Center.
READ MOREJune

Under Principal Investigator LTC Christopher J. Colombo, MD at Madigan Army Medical Center, a Geneva team is selected to rapidly develop and deploy a National Telecritical Care Network (NETCCN) for COVID-19 to bring critical care resources to every bedside.
READ MORESeptember

Geneva is selected as a sub under prime contract research organization (CRO) Pharm-Olam to administer phase III clinical trials at six military treatment facilities for two lead COVID-19 vaccine candidates being developed under Operation Warp Speed (OWS). OWS is a public-private partnership to deliver safe and effective doses of a COVID-19 vaccine by January 2021.
READ MOREOctober

With the addition of 4,037 square feet at its offsite facility to support the On-Demand Blood program, the USU-4D Bio3 Center facility expands to 10,267 square feet and includes a biofabrication suite, tissue culture room, bioreactor and sensor facility, biotechnology incubator suite, medical/surgical simulation room, and distance education and training facility.
READ MORENovember

Andriy Batchinsky, MD is awarded Geneva’s Researcher of the Year. As a leader in military medicine, Dr. Batchinsky is pioneering new treatments for trauma care and lung injuries both on and off the battlefield.
READ MOREDecember
Geneva Principal Investigator Sheli Radoshitzsky, PhD at USAMRIID, et al., demonstrates proof of concept for an immunotoxin-based targeted killing of infected cells as a potential antiviral intervention for the Ebola virus.
READ MOREAt the Heart of Geneva is Our Employees
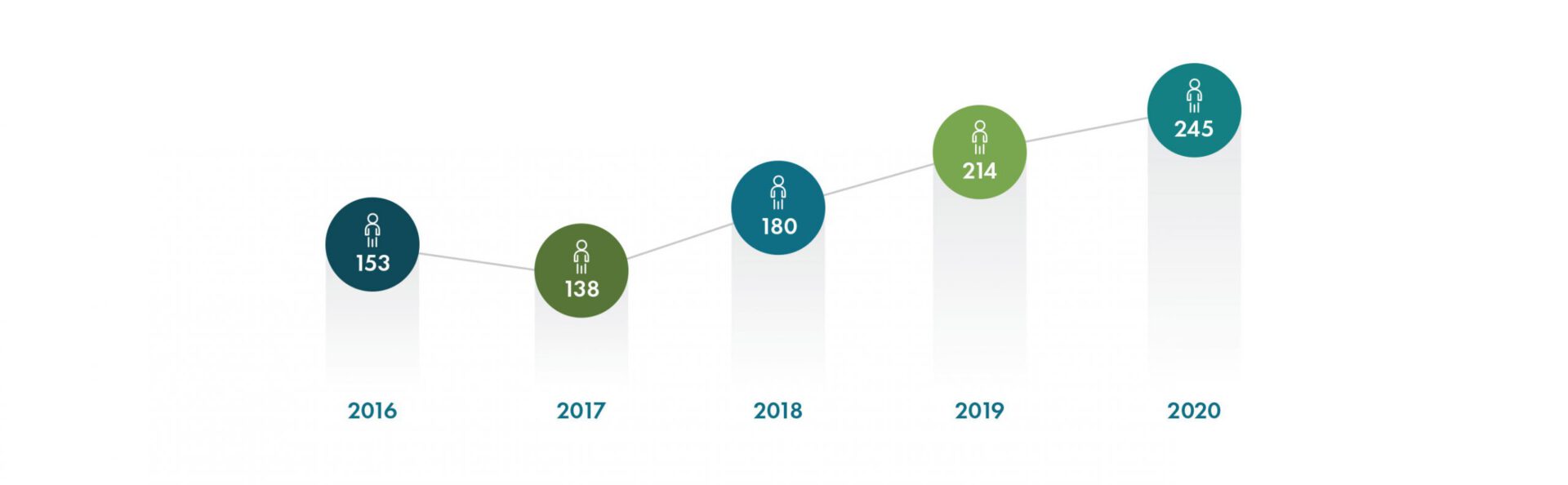
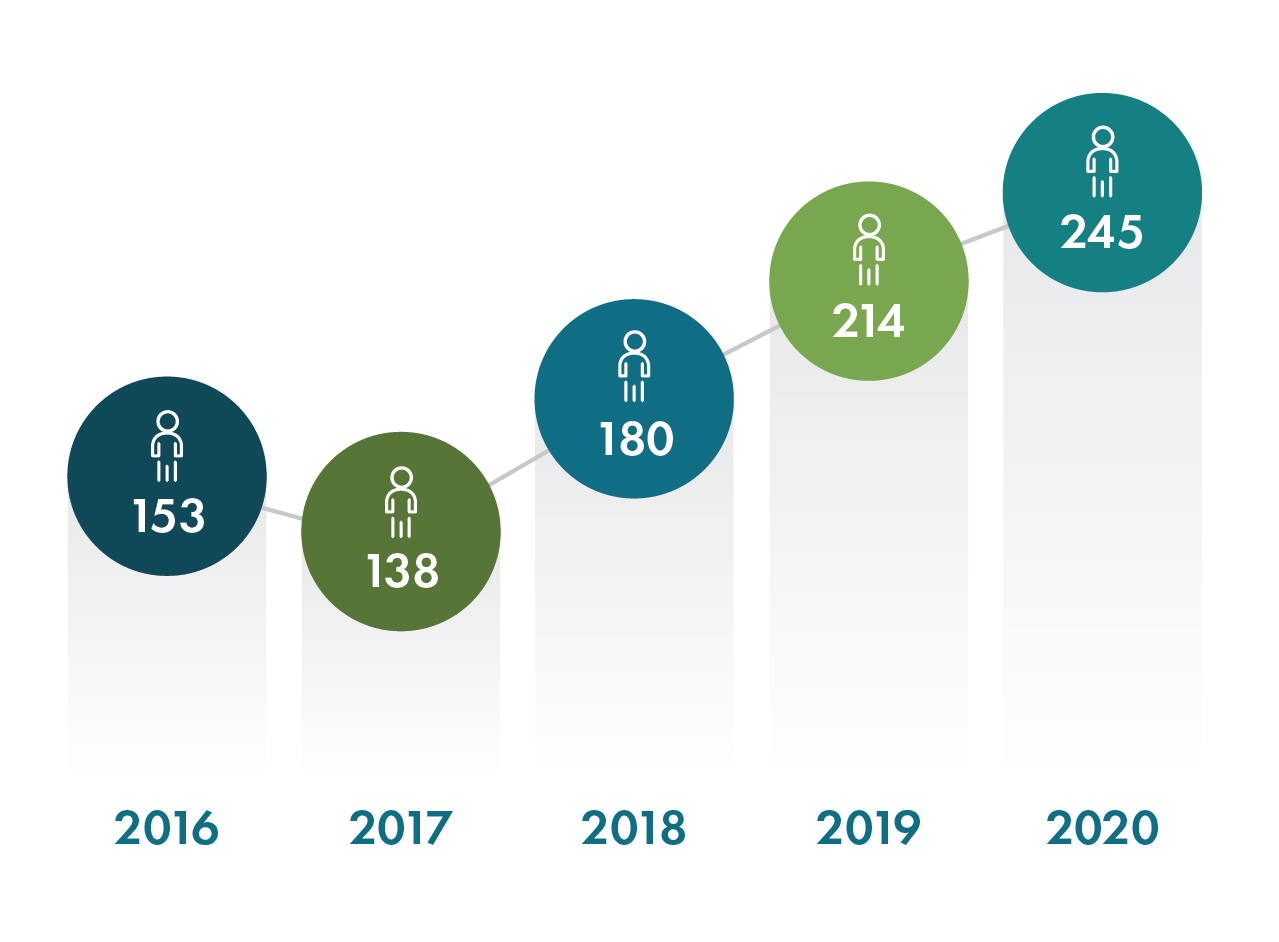
With a record number of new hires added, including over 115 employees to support Operation Warp Speed, Geneva employed over 550 people worldwide at the end of 2020. We are tremendously grateful for the dedication and resilience of our team, who quickly pivoted to address the many challenges presented during the COVID-19 pandemic.
New Hires
Highly Skilled and Technical Employees in the Field
Geneva employs over 460 highly-skilled and technical research professionals at 50+ performance sites across the world. Geneva’s experience in hiring and retaining a variety of these positions proved to be invaluable as the urgent needs of COVID-19 research were felt across the organization.


Geneva’s Wave the Flag Recipients
Geneva recognizes excellence in employees who go above and beyond the normal scope of their work, demonstrate Geneva’s core values, and strengthen our mission of promoting and supporting the advancement of military medicine. The Wave the Flag award is presented quarterly to an employee who exceeds expectations and exhibits Geneva’s core values.