Our COVID-19 Research
Tackling Challenges from Many Angles
Geneva is well-positioned to continue the fight against COVID-19. As a leading organization that drives military medical research around the world, Geneva is grounded in experience with a 28-year history of success. Using knowledge gained from research addressing global infectious disease threats, our infectious disease research – combined with our scientific and technical program management expertise – puts Geneva in a unique position to advance COVID-19 research.
VACCINE DEVELOPMENT
Clinical Trials
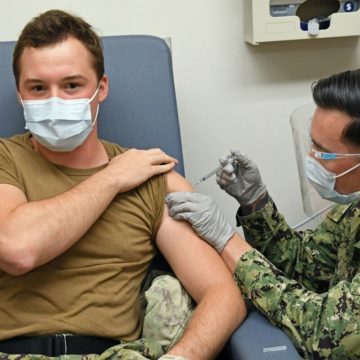
Geneva has advanced phase III clinical trials for two COVID-19 vaccines at six MTFs and supports additional trials under NIH-sponsored ACTIV-2 and ACTIV-4 master protocols to speed the development of the most promising COVID treatments and vaccines. Geneva is also conducting an observational study of active duty service members who have been vaccinated.
NOVEL THERAPEUTICS
Monoclonal Antibodies
Using human monoclonal antibodies isolated from survivors, Geneva researchers are advancing promising new treatments for COVID-19 and advancing novel applications to minimize viral replication.
RAPID DIAGNOSTICS
Biosurveillance
Leveraging technology capabilties, Geneva researchers are developing a multiplex remote system for biosurveillance to diagnose viruses, including SARS-CoV-2, using a readily-available smartphone.
COUNTERMEASURES
Treatment of Co-Morbidities
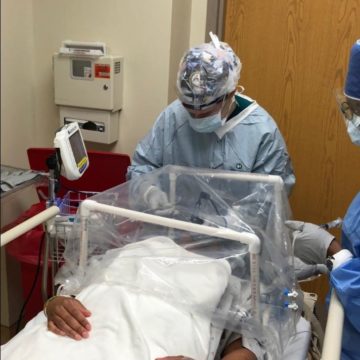
Geneva researchers are studying ways to limit the duration of patients suffering from acute respiratory distress syndrome (ARDS) caused by COVID-19, contributing to post-COVID best practices, and understanding how the virus is passed in placenta from the mother to baby before and at birth.
Featured Program
USING TELEMEDICINE TO BRING CRITICAL CARE SUPPORT TO EVERY BEDSIDE
Under the National Emergency Tele-Critical Care Network project (NETCCN), Geneva researchers are helping to address a critical problem for COVID healthcare: the shortage of critical care trained clinicians. NETCCN is an evolving network of clinical care teams that provide expert medical advice to anyone who needs it, wherever they may be, using network-enabled mobile devices.
Case Study
Operation Warp Speed
Operation Warp Speed (renamed the COVID-19 response operation in January 2021) and the multi-agency initiative’s central goal – to develop, produce, and distribute 300 million doses of a safe and effective coronavirus vaccine by January 2021 – required a deep knowledge of science and how to manage complex government operations while navigating a challenging pandemic. Prime contract research organization (CRO) Pharm-Olam and Geneva were selected by the Department of Defense to support phase 3 clinical trials for two vaccine candidates.
GENEVA’S ROLES
• Clinical trials management at military treatment facilities (MTFs)
• Regulatory expertise
• Scientific expertise
• Recruitment of highly skilled medical and technical personnel
• Facilitating the rapid acceleration of research timelines
Over 115 Geneva employees supported OWS with 1,720 total participants enrolled across six military treatment facilities nationwide.