4 March 2022
Why Diversity Matters in Clinical Trials
Clinical trials are essential to medical innovation, improved patient outcomes, and the generation of invaluable scientific data. However, they are typically resource-intensive and require adequate recruitment and retention rates to achieve sufficient evidence and minimize bias. The U.S. Food and Drug Administration (FDA) states that overall, few people sign up for and participate in trials, and those who do participate aren’t always representative of the U.S. population.
The U.S. military population, made up of active duty personnel, retirees, their families, and veterans, is a highly diverse and compliant patient population that offers specific benefits to clinical trials not always available in civilian populations.
Importance of Recruitment, Retention, and Diversity in Clinical Trials
Optimal participant recruitment and adequate retention rates are essential to ensuring meaningful results in any clinical trial, but participation overall is especially low for certain populations, including adults age 75 or older and people from certain racial and ethnic groups, according to the FDA.
Veterans see themselves as more disciplined (84%) and patriotic (71%) than those who have not served in the military. Participant compliance is essential to reduce study delays and improve outcomes of clinical trials.
It is also important for clinical trials to have participants of different ages, genders, races, and ethnicities. When clinical trials include diverse participants, the study results may have a much wider applicability. Studies have shown that there can be important differences in how people of diverse groups respond to medical products. According to Pfizer, genetics, gender, weight, ethnic origin, even geographic location – all of these may play a role in treatment efficacy and safety. Fortunately, the military community is well-represented in these key requirements.
Diversity in the U.S. Military
As the country has become more racially and ethnically diverse, so has the U.S. military. Racial and ethnic minority groups made up 40% of DoD active-duty military in 2015, up from 25% in 1990. Hispanics are the fastest-growing minority population in the military – a shift that aligns with larger demographic trends in the U.S.
The active-duty military population has also grown older, according to the Pew Research Center. Roughly two-thirds of all DoD active-duty military personnel were ages 30 or younger in 2015. One-in-ten (9%) were older than 40, representing a steady increase in age over the last 40 years.
Diversity Results Highlight: Operation Warp Speed
Geneva was selected as a subcontractor under prime contract research organization (CRO) PharmOlam to administer phase III clinical trials at six military treatment facilities for two lead COVID-19 vaccine candidates being developed under Operation Warp Speed (OWS). OWS was a partnership created to deliver safe and effective doses of a COVID-19 vaccine by January 2021. OWS was later renamed COVID-19 Response Operation.
AstraZeneca
Geneva enrolled 1,447 participants at five military treatment facilities.
- 24% 65+ years and older
- 55% had co-morbidities
- 10% AA/Black
- 11% LatinX
- 72% Caucasian
Novavax
Geneva enrolled 240 participants at one military treatment facility.
- 30% older than 65
- 25% AA/Black
- 5% Asian
- 8% LatinX
- 68% Caucasian
Geneva has over 28 years of experience in advancing clinical trials within the Department of Defense (DoD)’s network of military treatment facilities. Geneva is fully integrated within the Military Health System and delivers an average of 75 open industry-sponsored studies at any given time, with an estimated 25 new industry-sponsored studies per year. Contact Geneva to learn more about our clinical trials capabilities.
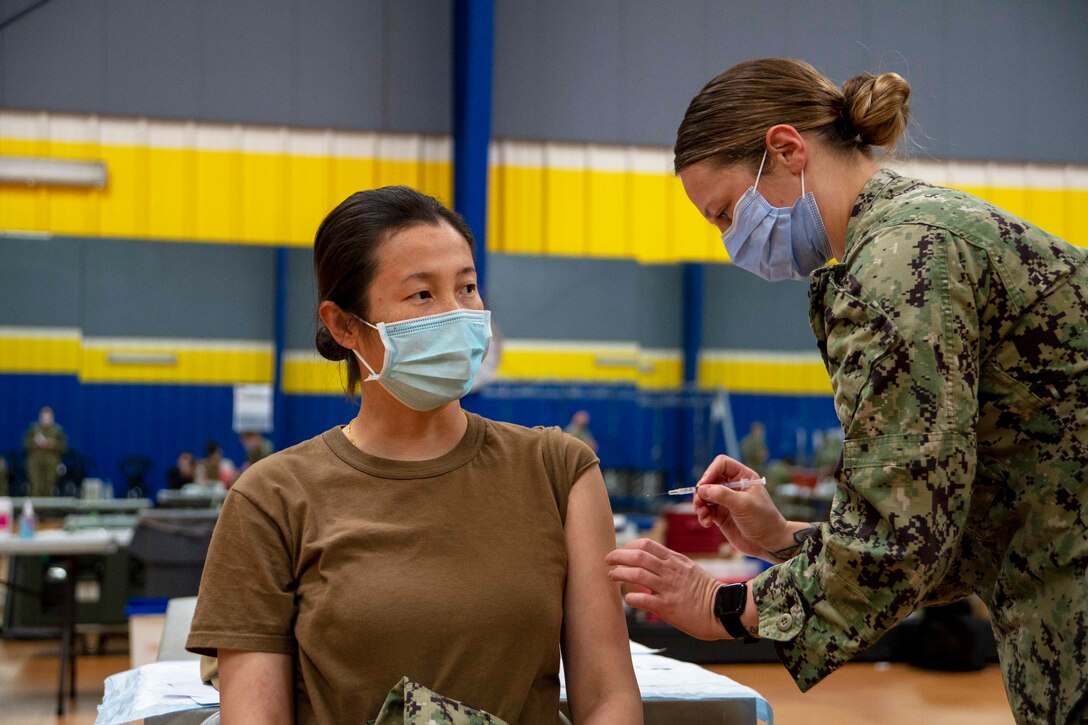
Genetics, gender, weight, ethnic origin, even geographic location – all of these may play a role in treatment efficacy and safety.
HIGHLIGHTS
- The U.S. military population, made up of active duty personnel, retirees, their families, and veterans, is a highly diverse and compliant patient population that offers specific benefits to clinical trials not always available in civilian populations.
- When clinical trials include diverse participants, the study results may have a much wider applicability.
- Geneva is fully integrated within the Military Health System and delivers an average of 75 open industry-sponsored studies at any given time, with an estimated 25 new industry-sponsored studies per year.


