7 July 2022
7 Ways Geneva is Advancing #MilMed in the National Capital Region
The National Capital Region (NCR) military medical market includes all of the federal district and parts of the U.S. states of Maryland and Virginia. This area is more widely known as the Washington, DC metropolitan area. Encompassing the majority of federal agencies, the NCR is crucial to military medical research infrastructure.
Recognizing the need to expand its corporate presence and continue growing its portfolio of military medical research, Geneva opened its NCR office in Bethesda, Maryland in 2018.
Geneva’s mission and services are centered around advancing military medical research in collaboration with top military researchers and innovators, key industry leaders, and collaborators in a variety of research areas – many of which are located in the NCR.
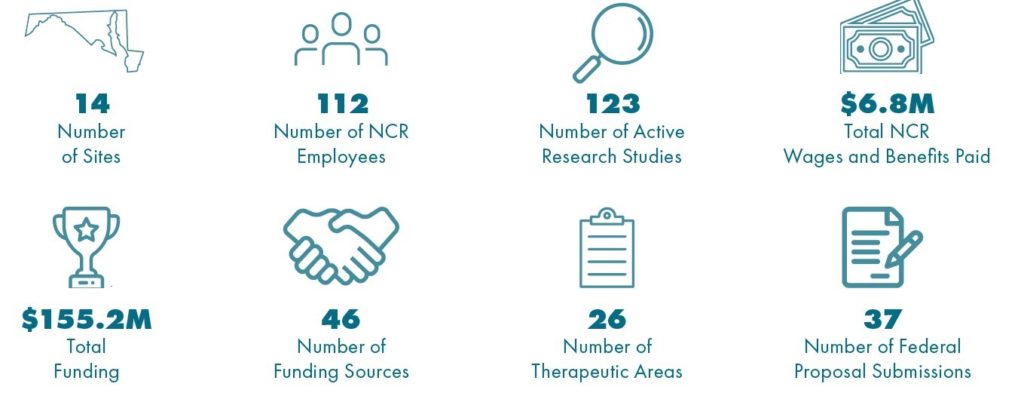
Geneva’s 2021 Impact in the National Capital Region
Here are 7 performance sites located in the NCR where Geneva is managing critical research programs to ensure optimal health for service members and the communities they serve.
1. USU-4DBio3 Center for Biotechnology, Bethesda, MD
The 2014 National Defense Authorization Act was signed into law includes language to officially extend privileges to Geneva to work directly with the Uniformed Services University (USU). Since that time, Geneva entered into an MOU with USU to collaborate on multiple activities for the purposes of conducting medical research and education within the DoD and nationwide.
In 2017, USU issued a cooperative agreement to Geneva to facilitate the execution of the 4D Biofabrication, Bioprinting, and Biomanufacturing (4D Bio³) program which would later become the USU-4DBio³ Center for Biotechnology. In 2019, USU issued a cooperative agreement to Geneva to facilitate the execution of the Musculoskeletal Injury Rehabilitation Research for Operational Readiness (MIRROR) program.
Performance Highlights
- Fabrication for Austere Military Environments (FAME) – The FAME program within the USU-4D Bio³ Center for Biotechnology is developing convergent manufacturing austere solutions for operational environments. “Bullets to Biotech” includes a multi-material 3D printer that is deployed into multiple environments to provide real-time solutions and new logistical capabilities for warfighter/humanitarian use including a Role 2 desert location and International Space Station.
- On-Demand Blood (ODB) – Using state-of-the-art biotechnology, ODB targets the production of human red blood cells and neutrophils, with the intent of producing full range of clean human blood products on-demand (“whole blood”) in austere environments for military, global health, and disaster use.
- Musculoskeletal Injury Rehabilitation Research for Operational Readiness (MIRROR) – MIRROR delivers high value research, education, training, and infrastructure for over 40 clinically relevant musculoskeletal injury (MSI) studies within the military health system. MIRROR supports a broad scope of projects, including epidemiological investigation, investigator-initiated pilots, and prospective randomized multisite clinical trials. Results of these studies generate evidence-based approaches for future clinical practice guidelines as well as educational opportunities for future military and civilian providers.
2. U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), Fort Detrick, MD
Geneva has advanced innovative research at Fort Detrick for 10+ years. Geneva is actively managing 26 research programs in infectious diseases at USAMRIID.
Performance Highlights
- Mammalian and Plant-derived Antibody-based Therapies against Sudan Ebolavirus – The objective of this six-year National Institutes of Health-funded project was to develop an antibody-based immunotherapy for the treatment and management of Sudan virus (SUDV) infection to address a gap in the current Ebola virus therapeutics portfolio. The gap was identified as particularly concerning as the causative agent of three of the last four Ebola virus outbreaks was SUDV. The USAMRIID/Geneva team successfully created a monoclonal antibody that is being further developed.
- Therapeutic Molecule Phenotypic Screening Capability – Geneva is contracted to manage therapeutic molecule phenotypic screening on behalf of USAMRIID using a high content imaging (HCI) platform. The effort includes developing new SARS-CoV-2 cell-based screens as well as collecting, analyzing, and reporting screening data and results to the US Government for support of additional coronavirus therapy discovery and development projects.
3. Telemedicine & Advanced Technology Research Center (TATRC)
Geneva is actively managing two research programs in federal contracts, telemedicine, and rehabilitative medicine at TATRC. This research is led by two principal investigators and industry collaborators such as Massachusetts General Hospital, DocBox, Omnicure, the Society for Critical Care Medicine, and Google.
Performance Highlight
Geneva was awarded a contract from 2011-2017 to provide medical research and project management support for TATRC, a subordinate of the U.S. Army Medical Research & Development Command (USAMRMC) charged with performing medical reconnaissance and special operations to address critical gaps in under-represented DoD medical research programs. Geneva employed 16 research management, technical, and/or scientific support personnel for TATRC at Fort Detrick, MD and Fort Gordon, GA and other locations.
4. Walter Reed Army Institute of Research (WRAIR), Silver Spring, MD
In 2005, Geneva was awarded its first federal contract award to establish a Sponsored Research Office at WRAIR and in 2006, Geneva received its first National Institutes of Health awards at WRAIR in the areas of respiratory toxicity and lung injury. Geneva manages 16 active programs at WRAIR for 13 principal investigators at WRAIR.
Performance Highlight
Principal Investigator Damon Ellison, PhD, Chief of Bacteriophage Therapeutics at WRAIR, is part of a research collaboration evaluating an investigational live recombinant measles-vectored chikungunya vaccine in previously exposed adults. The Phase 2 clinical trial began in July 2019 with 21- to 50-year old subjects enrolled, followed by subjects aged 51 to 65 pending a review of favorable safety data.
5. National Cancer Institute (NCI), Bethesda and Frederick, MD
Geneva is actively managing 18 NCI Campus research programs in oncology at NCI.
Performance Highlight
In 2015, Geneva awarded its first DoD Joint Warfighter Medical Research Program contract to support 2015 Geneva Researcher of the Year LTC Luis Alvarez, PhD, in regenerative medicine with work accomplished at NCI-Frederick. This contract focused on stem-cell based tissue engineering approaches to address loss of function resulting from combat trauma and disease.
6. TriService Nursing Research Program (TSNRP), Bethesda, MD
In 1996, Geneva received its first nursing research grants from TSNRP. These grants were Geneva’s first federally funded research programs and launched a rewarding collaboration with the nursing researchers at Madigan Army Medical Center that continues to this day. Since that time, Geneva has remained committed to military nursing research in a broad spectrum of military treatment facilities across the nation.
In 2019, Geneva assumed a program management role over TSNRP under a cooperative development agreement with USU. Geneva oversees the award making and award management processes for TSNRP. As the program management office of the TSNRP, Geneva manages the full grant making lifecycle. TSNRP is the only funding entity for military nurse researchers to advance nursing science and patient care.
7. Fort Belvoir Community Hospital, Fort Belvoir, VA
Geneva manages 18 research programs at Fort Belvoir Community Hospital at Fort Belvoir, Virginia in areas such as behavioral and mental health, emergency medicine, health informatics, immunology, infectious diseases, neurology, and oncology.
Performance Highlight
Principal Investigator Navy Commander Christina La Croix, DO is collaborating with Geneva on a prospective, longitudinal study with the goal of establishing a database to provide needed empirical information on the chronic and late-life effects of mild traumatic brain injury (mTBI), including those that may stem from neurodegeneration. The research aims to identify predictors of and early, preclinical evidence of neurodegeneration, to assist in the prognosis, development, and targeted application of neuroprotective therapy. This protocol is one of many large-scale trials included in the Long-Term Impact of Military-Relevant Brain Injury Consortium – Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC).
Geneva recognizes that collaboration is vital to discovery and advancement. We welcome the opportunity to partner with those who share our commitment to accelerating medical breakthroughs, improving the lives of military and civilian populations worldwide.
Disclaimer: The views expressed do not reflect the official policy of the Army, the Department of Defense, or the U.S. Government.

Recognizing the need to expand its corporate presence and continue growing its portfolio of military medical research, Geneva opened its NCR office in Bethesda, Maryland in 2018.
HIGHLIGHTS
- Military medical research conducted in the NCR is critical to ensuring optimal health for service members and the communities they serve.
- Geneva collaborates with 14 top federal laboratories and military treatment facilities in the NCR, employing 112 people working on over 120 research studies in 26 therapeutic areas.
- Geneva recognizes that collaboration is vital to discovery and advancement. We welcome the opportunity to partner with those who share our commitment to accelerating medical breakthroughs, improving the lives of military and civilian populations worldwide.


